PIPAC Treatment: Success Rates & Statistics
PIPAC Treatment: Success Rates, Statistics, and Everything You Need to Know
Peritoneal carcinomatosis, a condition where cancer spreads to the lining of the abdominal cavity, has historically been challenging to treat. However, Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) has emerged as a promising treatment option. This innovative approach has shown encouraging results in improving the quality of life and survival rates for patients with advanced abdominal cancers. In this comprehensive guide, we’ll explore what PIPAC is, its global adoption, current clinical trials and success rates.
What is PIPAC?
PIPAC is a minimally invasive treatment for peritoneal carcinomatosis, a condition often associated with cancers of the gastrointestinal tract, ovaries, and other abdominal organs. Unlike traditional chemotherapy, which is delivered intravenously, PIPAC administers chemotherapy directly into the abdominal cavity as an aerosol under pressure. This method allows for a more targeted delivery of the drug, ensuring higher concentrations reach the cancerous tissues while minimizing systemic side effects.
The procedure is performed laparoscopically, meaning it requires only small incisions. A surgeon inserts a nebulizer into the abdominal cavity, which converts liquid chemotherapy into a fine mist. The pressurized environment ensures the mist penetrates deeply into the peritoneal lining, where cancer cells are located. PIPAC is typically repeated every 4-6 weeks, depending on the patient’s response and overall health.
How Long Has PIPAC Been Used, and Where?
PIPAC was first introduced in 2011 by Professor Marc Reymond and his team in Germany. Since then, it has gained traction worldwide as a viable treatment option for peritoneal carcinomatosis. Over the past decade, PIPAC has been adopted in numerous countries, including:
- Europe: Spain, Italy, Germany, France and the UK have been at the forefront of PIPAC adoption. Many leading cancer centers in these countries now offer PIPAC as part of their treatment protocols.
- Asia: Countries like India, South Korea, and Japan have also started incorporating PIPAC into their oncology practices.
- North America: While PIPAC is still in the early stages of adoption in the United States and Canada, several clinical trials are underway to evaluate its efficacy and safety.
- Australia: Australian medical institutions have also begun exploring PIPAC as a treatment option for peritoneal carcinomatosis.
The growing global interest in PIPAC is a testament to its potential to improve outcomes for patients with advanced abdominal cancers.
Overview of Current Clinical Trials and Their Results
Clinical trials are essential for evaluating the safety, efficacy, and long-term outcomes of PIPAC. Over the past few years, numerous studies have been conducted to assess its impact on survival rates, symptom relief, and quality of life. Here’s an overview of some key findings:
-
Survival Rates:
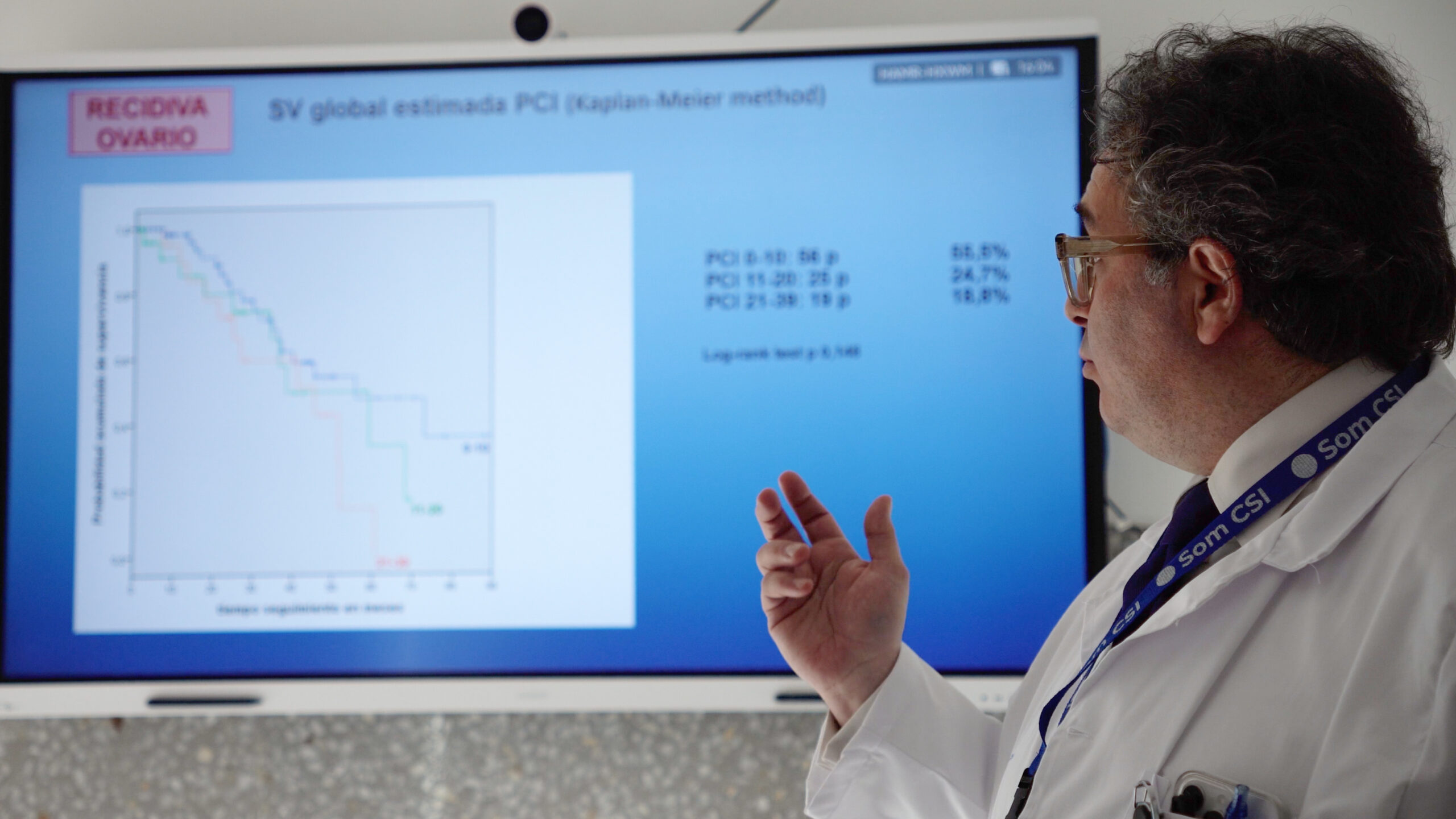
A 2020 study published in the Annals of Surgical Oncology reported that PIPAC improved overall survival in patients with peritoneal carcinomatosis from gastric cancer. The median survival rate increased from 6 months to 15 months in patients who underwent PIPAC combined with systemic chemotherapy.
Another study focusing on ovarian cancer showed that PIPAC led to a significant reduction in tumor burden and improved progression-free survival in patients with recurrent disease. -
Symptom Relief and Quality of Life:
PIPAC has been shown to alleviate symptoms such as abdominal pain, bloating, and ascites (fluid buildup in the abdomen). Patients often report improved quality of life after undergoing PIPAC, as the procedure is less invasive and has fewer side effects compared to traditional chemotherapy. -
Combination Therapies:
Researchers are exploring the use of PIPAC in combination with other treatments, such as cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC).
Early results suggest that combining PIPAC with these therapies may enhance overall outcomes. -
Ongoing Trials:
As of 2023, there are over 50 clinical trials registered worldwide investigating various aspects of PIPAC, including its use in different cancer types, optimal dosing regimens, and long-term safety.
These trials aim to establish standardized protocols and expand the evidence base for PIPAC.
While the results are promising, it’s important to note that PIPAC is still considered an emerging therapy. More large-scale, randomized controlled trials are needed to confirm its benefits and identify potential limitations.
PIPAC Success Rates: What Do the Statistics Say?
The success rates of PIPAC vary depending on the type and stage of cancer, the patient’s overall health, and other factors. However, several studies have reported encouraging statistics:
-
Response Rates: Studies have shown that PIPAC achieves a histological response (reduction in cancer cells) in 60-80% of patients with peritoneal carcinomatosis.
-
Tumor Regression: In some cases, PIPAC results in tumor regression and allows re-evaluation of the possibility of complete surgical treatment (cytorreductive surgery with HIPEC).
-
Long-Term Outcomes: While long-term survival data is still limited, early results suggest that PIPAC can extend survival by several months to years in certain patient populations.
It’s worth noting that PIPAC is not a cure for peritoneal carcinomatosis. Instead, it is often used as a palliative treatment to control symptoms and improve quality of life. In some cases, it may be combined with other therapies to achieve better outcomes.
Every Medical Case is Unique: The Importance of Consulting a Specialist
While the statistics and clinical trial results are encouraging, it’s crucial to remember that every medical case is unique. Factors such as the type and stage of cancer, the patient’s overall health, and previous treatments can all influence the effectiveness of PIPAC.
If you or a loved one is considering PIPAC, it’s essential to consult with a specialized oncologist or surgeon who has experience with this procedure. They can provide personalized advice based on your specific condition and help you weigh the potential benefits and risks.
Conclusion
PIPAC represents a significant advancement in the treatment of peritoneal carcinomatosis, offering hope to patients with limited options. Its minimally invasive nature, targeted drug delivery, and promising success rates make it a valuable addition to the oncology toolkit.
However, as with any medical treatment, it’s important to approach PIPAC with realistic expectations and under the guidance of a qualified specialist.
If you’re interested in learning more about PIPAC or exploring whether it’s the right option for you, don’t hesitate to contact us and we will answer you as soon as possible.
